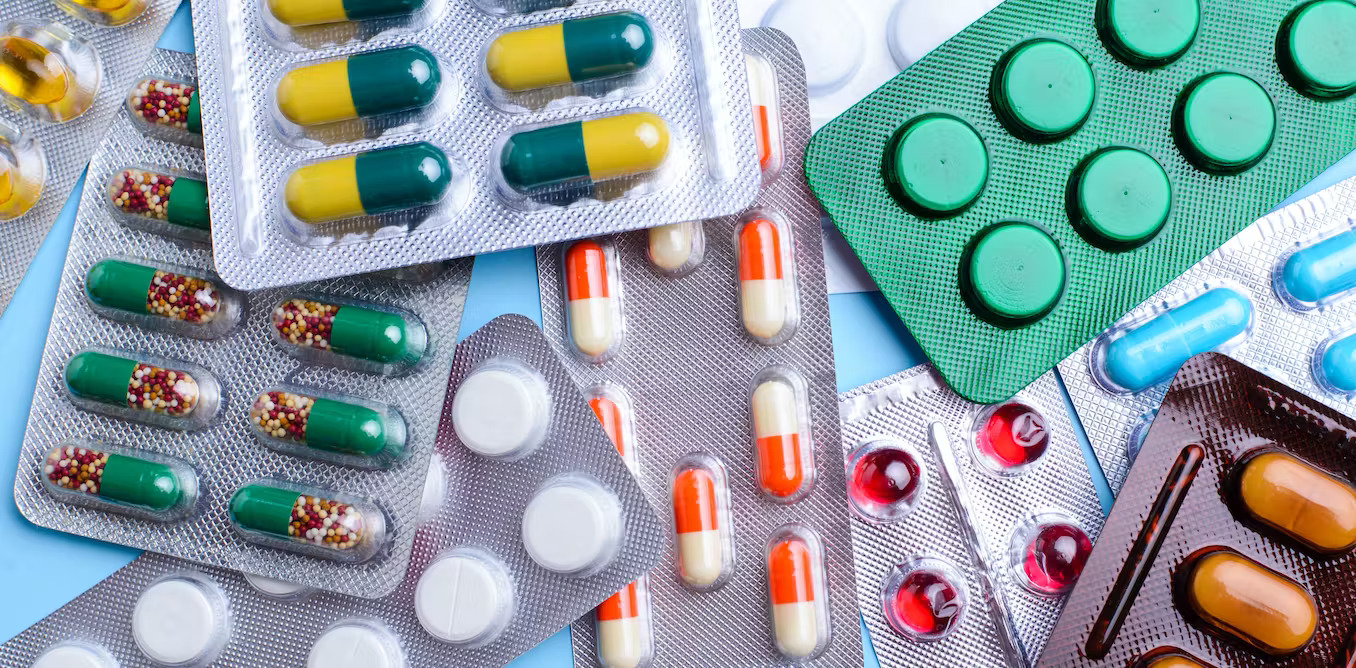
DRUGS & PHARMACEUTICALS -IP BP USP EP & JP
 Bombay Test House (BTH), is a State of the Art Commercial and Public Testing Laboratory (PTL) approved by FDA (Food & Drug Administration) - MAHARASHTRA for Drugs, Pharmaceuticals and Cosmetics Testing & Analysis.
Bombay Test House (BTH), is a State of the Art Commercial and Public Testing Laboratory (PTL) approved by FDA (Food & Drug Administration) - MAHARASHTRA for Drugs, Pharmaceuticals and Cosmetics Testing & Analysis.
 BTH is equipped with highly sophisticated Instruments such as HPLC , GC MS, ICP OES, UV VIS, FTIR, and having Accreditations, Recognitions and Approvals from various National and International bodies, is entirely independent and is not associated with any Corporations, Industries, Importers and Exporters or any of the Govt. bodies.
BTH is equipped with highly sophisticated Instruments such as HPLC , GC MS, ICP OES, UV VIS, FTIR, and having Accreditations, Recognitions and Approvals from various National and International bodies, is entirely independent and is not associated with any Corporations, Industries, Importers and Exporters or any of the Govt. bodies.
Our clientele is extensive which includes Major Corporations, Multinational Companies, Public Sector as well as Private Sector Companies and other Government Agencies.
 Bombay Test House (BTH) now an ISO/IEC 17025: 2017 NABL Accredited Laboratory and ISO 9001, 14001 & 45001 Certified Lab, offers full Physical, Chemical, Instrumental and Microbiological testing and analyse products from a wide variety of Industries.
Bombay Test House (BTH) now an ISO/IEC 17025: 2017 NABL Accredited Laboratory and ISO 9001, 14001 & 45001 Certified Lab, offers full Physical, Chemical, Instrumental and Microbiological testing and analyse products from a wide variety of Industries.
 Our testing team comprises of competent professionals, viz., Doctorates, Geologists, Engineers,
Pharmacist, Chemists and Microbiologists have decades of experience, who are experts in IP, BP, USP, JP and Chinese Pharmacopeia besides FSSAI, BIS, AOAC,ASTM, APHA,ISO, DGHS and other National and International methods of Analysis.
Our testing team comprises of competent professionals, viz., Doctorates, Geologists, Engineers,
Pharmacist, Chemists and Microbiologists have decades of experience, who are experts in IP, BP, USP, JP and Chinese Pharmacopeia besides FSSAI, BIS, AOAC,ASTM, APHA,ISO, DGHS and other National and International methods of Analysis.
Drugs & Pharmaceutical Testing:
 Welcome to Bombay Test House Pvt Ltd., Food and Drug Administration ( FDA-MH), Form-37
Govt. of Maharashtra Approved GLP Certified Drugs & Pharmaceutical Testing Laboratory!
Welcome to Bombay Test House Pvt Ltd., Food and Drug Administration ( FDA-MH), Form-37
Govt. of Maharashtra Approved GLP Certified Drugs & Pharmaceutical Testing Laboratory!
We are a state-of-the-art facility dedicated to providing the comprehensive and highest quality testing services for the pharmaceutical industry. Our laboratory is equipped with the latest technology and experienced staffed by highly trained professionals have the expertise and are committed to analyse and certify the safety and quality of your products.
At Bombay Test House Pvt Ltd., we offer a broad range of testing services including chemical analysis, physical testing, microbiological testing, and stability testing. We are committed to providing accurate and reliable results in a timely manner. Our laboratory is ISO/IEC 17025 accredited and ISO 9001, 14001 & 45001 Certified and our testing procedures adhere to the highest industry standards.
We also offer traceability and verification services for our clients. We understand that the safety and quality of your products can have a direct impact on the health and well-being of your customers. That is why our team of highly trained and experienced professionals work diligently to ensure that your products meet the highest safety and quality standards.
At BTH Pharmaceutical Testing Laboratory, we are committed to providing reliable results, timely customer service, and value-added services. Thank you for considering us for your testing needs. Please feel free to contact us with any questions you may have.
The following are the Testing and Analysis Services offered by Bombay Test House Pvt Ltd. for Drugs & Pharmaceutical Industry.
- Analytical Testing: We offer a wide range of analytical testing services, including chemical analysis, , microbiological analysis, and stability testing. We can test raw materials, finished products, and in-process materials to ensure they meet the required specifications.
- Instrumental Analysis: Refers to the use of scientific instruments to measure and analyse chemical compounds and substances. These instruments can include Spectrophotometers, Chromatographs, Mass Spectrometers, and other specialized equipment.
- Microbial Testing: We use the latest techniques to identify and quantify microorganisms in your products. We can detect bacteria, fungi, and other microorganisms that can affect the safety and efficacy of your products.
- Stability Testing: We can help you determine the shelf life of your products by conducting stability testing under various conditions. We can test your products for changes in appearance, pH, potency, and other parameters over time.
- Regulatory Support: We can help you navigate the complex regulatory landscape of the pharmaceutical industry. We can provide guidance on compliance with FDA, EU, and other regulations, and help you prepare for inspections and audits.
- Consultancy Services: Our team of experts can provide advice and guidance on all aspects of pharmaceutical testing, from method development to data interpretation.
Categories of drugs:
-
Drugs other than those specified in Schedule C and C(1) and also excluding
Homeopathic Drugs:-
- Crude Vegetable Drugs
- Mechanical Contraceptives
- Surgical Dressing
- Drugs requiring the use of Ultra Violet/Infra-Red. Or Chromatography
- Disinfectants
- Other Drugs
-
Drugs Specified in Schedule C and C (1)
- Sera, Vaccines, Antigens, Toxins, Anti Toxins, Toxoids, Bacteriophages and Similar Immunological Products.
- Antibiotics
- Vitamins
- Parenteral Preparations
- Sterilized Surgical Ligature / Suture
- Drugs requiring Microbiological Tests Drugs requiring the use of Ultraviolet/Infra-Red/Spectrophotometer or Chromatography
- Other drugs
Bombay Test House Pvt Ltd ., undertakes Testing & Analysis of Various:
Pharmaceutical Bulk Drugs (API). Drug intermediates etc.,
Finished Pharmaceutical Formulations such as
- Tablets
- Capsules
- Liquid orals
- Injectable
- Topical
- Ophthalmic
- Nasal
- Inhalers and other products
| EXPERTISE AND ALLIED SERVICES | |||
|---|---|---|---|
| Analytical Research | IP/BP/USP/EP/JP | Impurity profile | Organic Volatile impurities(OV) |
| identification of various druge | Chromatographic purity/Related substancos | Residual solvents | |
| Purity of pharmaceuticals | Organic & Inorganic | Dissolution Profile & Content Uniformity | |
| AVAILABLE FACILITIES OF MAJOR EQUIPMENTS | ||||
|---|---|---|---|---|
| Major Equipments | GC MS / GC MS MS FID / Head Space/ECD/NPD/FPD | Differential Scanning Calorimetry (DSC) | Particle Size Malvern Mastersizer 2000 | CHNS(O) Analyzer |
| HPLC / LC MS MS | Thermo Gravimetric Analyzer (TGA) | Surface Area by BET Analyser | Brook Field Viscometer | |
| FTIR | Particle Size Analyzer - Malvern 2000 | XRD / XRF | Auto Titrator & KF Titrator | |
| ICP-OES / ICP MS | Nuclear Magnetic Resonance Spectroscopy (NMR) | TOC Analyzer | Surface Tensiometer - Platinum Ring Method | |
| UV-Vis Double Beam Spectrophotometer | Gel Permeation Chromatography (GPC) | Scanning Electron Microscopy (SEM) | Melting Point / Boiling Point Apparatus | |
| Microwave Digester | Ion Chromatography (IC) | Transmission Electron Microscopy (TEM) | Specific Optical Rotation - Automatic Polarimeter | |
| ANALYSIS OF DRUGS, PHARMACEUTICALS, COSMETICS & MICROBIOLOGICAL | ||||
|---|---|---|---|---|
| BIOLOGICAL DISCIPLINE | ||||
| Drugs and Pharmaceuticals | Antibiotics | Drug Intermediates | Microbial limit test | Synthetic Drugs |
| Bioassays of other products | Drug Substances (API) | Raw Materials | Veterinary Drugs | |
| Chemotherapeutic Agents | Endotoxins | Sterility Tests | Others | |
| Microbial Testing | Microbial Limit Tests | Sterility | Bacterial Endotoxin test | Yeast & Mould |
| Total Bacterial Count | Vitamin Assays | Antimicrobial Preservative Efficacy Test | Antibiotic Assays | |
| CHEMICAL DISCIPLINE | ||||
|---|---|---|---|---|
| Ayush Products | Ayurvedic Drugs | Homeopathic Drugs | Unani Drugs | |
| Herbal Formulations | Siddha Drugs | Others | ||
| Drugs & Pharamaceuticals | Capsules | Drops | Oral Liquids | Injectables |
| Creams | Gels | Oral Powders | Topical | |
| Drug Substance (API) | Ointment | Parenteral Preparations | Ophthalmic | |
| Nasal | Vitamis | |||
| Inhalers and other products | Tablets | |||
| Analytical Method Validation | Accuracy | Limit of Quantification | Range | |
| Precision | Limit of Detection | Robustness | ||
| Spacificity | Linerity | System suitability tests | ||
Testing
Read More
Inspection
Read More
Certification
Read MoreTesting Services
- Drugs & Pharmaceutical Testing
- Cosmetics & Essential Oils Testing
- Medical Devices Testing
- Ayush - Ayurvedic Drug Testing
- Food Products Testing
- Agri Commodities Testing
- Fertilizers and Soil Testing
- Animal Food & Feed Testing
- Water - Drinking Water & Effluent Water Testing
- Industrial Oils and Lubricants & Petroleum Products Testing
- Coal & Coke and Solid Fuels Testing
- Ores & Minerals Testing
- Metals & Alloys Testing
- Plastics, Polymer, Rubber & Rubber Products Testing
- Resins & Adhesives Testing
- Paints, Varnish, Pigments & Surface Coating Testing
- Glass & Ink, Paper & Pulp Testing
- Industrial & Fine Chemicals Testing
- Dyes, Acids and Solvents Testing
- Soaps, Detergents & Toiletries Testing
- Packaging & Packaging Products Testing
- Gold & Silver Assaying & Hallmarking
- Cement, Concrete & Building Materials Testing
- Pollution and Environmental Studies
- Microbiological Assays. Etc.,









