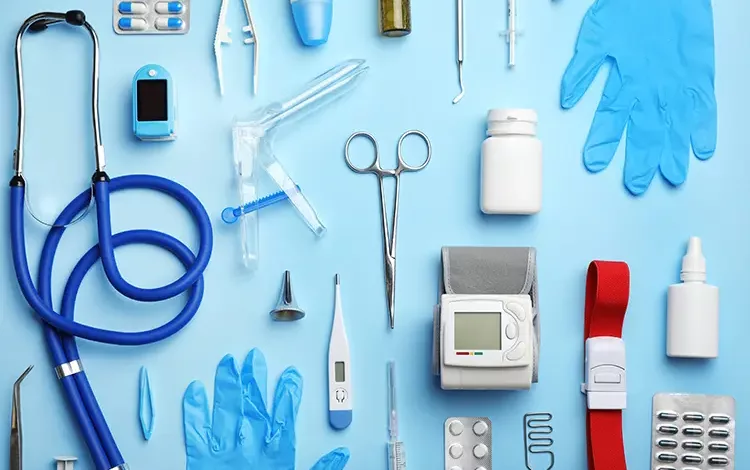
MEDICAL DEVICES TESTING:
Welcome to Medical Devices Testing as per MDR 2017.
 The Medical Devices Rules 2017 in India were implemented to regulate the import,
manufacture, distribution, and sale of medical devices in the country. These rules are
intended to ensure the safety and efficacy of medical devices and to protect the health of
patients.
The Medical Devices Rules 2017 in India were implemented to regulate the import,
manufacture, distribution, and sale of medical devices in the country. These rules are
intended to ensure the safety and efficacy of medical devices and to protect the health of
patients.
 The rules establish a regulatory framework for the classification, registration, and testing
of medical devices, and they also provide for the establishment of a central licensing and
registration system for medical device manufacturers and importers.
The rules establish a regulatory framework for the classification, registration, and testing
of medical devices, and they also provide for the establishment of a central licensing and
registration system for medical device manufacturers and importers.
 Additionally, the rules require that medical devices be labelled with specific information
and that they meet certain quality standards. Overall, the Medical Devices Rules 2017 aim
to ensure the availability of safe and effective medical devices in India.
Additionally, the rules require that medical devices be labelled with specific information
and that they meet certain quality standards. Overall, the Medical Devices Rules 2017 aim
to ensure the availability of safe and effective medical devices in India.
Medical devices are classified into four classes as per the Medical Device Regulation (MDR) 2017:
- Class I: Low risk devices, such as elastic bandages, that are subject to general safety and performance requirements.
- Class II(a): Devices that present a slightly higher risk than Class I devices, such as powered wheelchairs, and are subject to additional safety and performance requirements.
- Class II(b): Devices that present a higher risk than Class IIa devices, such as x-ray machines, and are subject to additional safety and performance requirements, as well as regulatory oversight by a notified body.
- Class III: Devices that present the highest risk, such as implantable pacemakers, and are subject to the most stringent safety and performance requirements, as well as regulatory oversight by a notified body.
- Class IV: Devices that present the highest risk and are subject to the most stringent safety and performance requirements, as well as regulatory oversight by a notified body.
All Medical Device must be CE Marked before it can be placed in the European market.
-
ISO (International Organization for Standardization) sets standards for medical devices
that help ensure their safety and performance. The specific testing parameters for a
medical device will depend on its classification and intended use, but some common
testing parameters for medical devices as per ISO regulations include:
- Biocompatibility: Testing to ensure that a device does not cause an adverse reaction when in contact with body tissue or fluids.
- Sterilization: Testing to ensure that a device can withstand the sterilization process without compromising its function or safety.
- Electrical safety: Testing to ensure that a device does not pose a risk of electric shock or fire.
- Mechanical safety: Testing to ensure that a device can withstand the forces and loads that it will be subjected to during normal use.
- Performance: Testing to ensure that a device performs as intended and meets its specified requirements.
- Software validation: Testing to ensure that the software used in a device is safe and functions correctly.
- Packaging: Testing to ensure that a device is properly packaged and protected during shipping and storage.
- Environmental: Testing to ensure that a device can withstand the environmental conditions in which it will be used.
- Labelling and instructions for use: Testing to ensure that the device is properly labelled and that the instructions for use are clear, accurate and easy to understand.
- Clinical evaluation: Testing to ensure that the device is safe and effective for its intended use by evaluating the device's performance in real-world use.
- The International Organization for Standardization (ISO) sets standards for medical
devices that help ensure their safety and performance. Some of the ISO standards related
to medical device testing include:
- ISO 10993-1:2018 "Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process" provides guidance on the selection of tests and the interpretation of results for the biological evaluation of medical devices.
- ISO 14155:2011 "Clinical investigation of medical devices for human subjects - Good clinical practice" sets guidelines for the conduct of clinical investigations of medical devices.
- ISO 14971:2019 "Medical devices - Application of risk management to medical devices" provides guidelines for the application of risk management to the development, production, and post-market surveillance of medical devices.
- ISO 15223-1:2016 "Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied" sets guidelines for the symbols that should be used on medical device labels and packaging.
- ISO 15223-3:2019 "Medical devices - Symbols - Part 3: Designation of single-use devices" sets guidelines for the symbols that should be used to indicate that a medical device is intended for single use.
- ISO 18113-1:2017 "Medical devices - Identification of in vitro diagnostic medical devices - Part 1: General requirements" sets guidelines for the identification of in vitro diagnostic medical devices.
- ISO 14644-1:2015 "Cleanrooms and associated controlled environments - Part 1: Classification of air cleanliness" provides guidelines for the classification of air cleanliness in cleanrooms and other controlled environments.
These standards provide the guidelines for the testing of the medical devices to ensure their safety and performance. The manufacturer must comply with the requirements of the standard before CE marking the device and placing it in the European market.
BESIDES, IN INDIA BUREAU OF INDIAN STANDARDS (BIS) HAS RELAESED A SET OF STANDRADRD TO BE FOLLOWING FOR MEDICAL DEVICES TESTING & ANALYSIS.
Testing
Read More
Inspection
Read More
Certification
Read MoreTesting Services
- Drugs & Pharmaceutical Testing
- Cosmetics & Essential Oils Testing
- Medical Devices Testing
- Ayush - Ayurvedic Drug Testing
- Food Products Testing
- Agri Commodities Testing
- Fertilizers and Soil Testing
- Animal Food & Feed Testing
- Water - Drinking Water & Effluent Water Testing
- Industrial Oils and Lubricants & Petroleum Products Testing
- Coal & Coke and Solid Fuels Testing
- Ores & Minerals Testing
- Metals & Alloys Testing
- Plastics, Polymer, Rubber & Rubber Products Testing
- Resins & Adhesives Testing
- Paints, Varnish, Pigments & Surface Coating Testing
- Glass & Ink, Paper & Pulp Testing
- Industrial & Fine Chemicals Testing
- Dyes, Acids and Solvents Testing
- Soaps, Detergents & Toiletries Testing
- Packaging & Packaging Products Testing
- Gold & Silver Assaying & Hallmarking
- Cement, Concrete & Building Materials Testing
- Pollution and Environmental Studies
- Microbiological Assays. Etc.,









