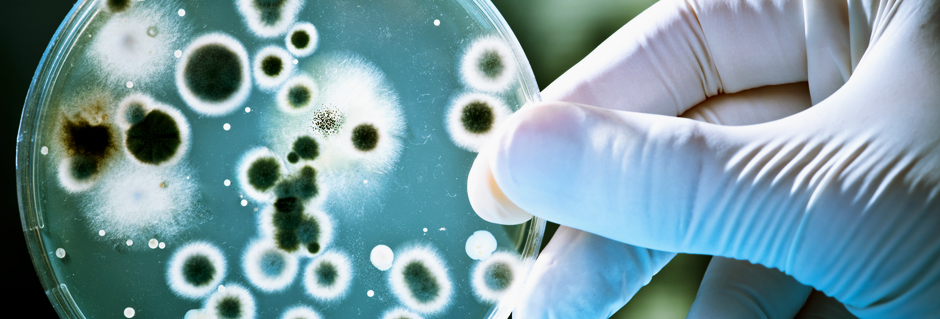
MICROBIOLOGICAL TESTING @ BOMBAY TEST HOUSE PVT LTD.
Microbiological assay and testing involve a series of tests and procedures to assess the presence, viability, and activity of microorganisms in various samples. Here are some common microbiological assay and testing protocols:
- 1. Microbial Identification: Identification of microorganisms to determine their species or strain using various techniques such as microscopy, biochemical tests, or molecular methods like polymerase chain reaction (PCR) or DNA sequencing.
- 2. Total Viable Count: Determines the total number of viable microorganisms present in a sample by performing colony-forming unit (CFU) counts on agar plates.
- 3. Sterility Testing: Tests for the absence of viable microorganisms in a product or sample, typically performed by inoculating the sample into growth media and observing for any microbial growth.
- 4. Environmental Monitoring: Regular monitoring of the microbial contamination levels in the production environment, including surfaces, air, water, and personnel, to ensure compliance with hygiene standards.
- 5. Antimicrobial Efficacy Testing: Evaluates the effectiveness of antimicrobial agents or disinfectants against specific microorganisms, typically performed using standardized methods such as the disk diffusion method or minimum inhibitory concentration (MIC) assays.
- 6. Preservative Efficacy Testing: Determines the ability of preservatives to inhibit the growth of microorganisms in various products, such as cosmetics, pharmaceuticals, or personal care products.
- 7. Pathogen Detection: Identifies the presence of specific pathogenic microorganisms, such as Salmonella, Escherichia coli, or Staphylococcus aureus, in samples using specific detection methods like immunoassays or molecular techniques.
- 8. Endotoxin Testing: Determines the presence and level of endotoxins, bacterial toxins derived from the cell wall of Gram-negative bacteria, using assays such as the Limulus amebocyte lysate (LAL) test.
- 9. Microbial Limits Testing: Determines the acceptable levels of microorganisms in a product or sample, typically performed by inoculating the sample into growth media and observing for microbial growth within specified limits.
- 10. Environmental Water Testing: Evaluates the microbial quality of water samples, such as drinking water, recreational water, or wastewater, to ensure compliance with safety and regulatory standards.
- 11. Validation and Verification: Validates and verifies the effectiveness of sterilization processes, sanitization procedures, or disinfection methods to ensure microbial control and safety.
- 12. Quality Control and Compliance: Microbiological assay and testing often involve following specific industry standards or regulations, such as those set by pharmacopeias (e.g., IP, BP, USP, EP) or regulatory bodies. Compliance with these standards ensures consistent quality, safety, and adherence to regulations.
The specific tests and protocols may vary depending on the type of sample, the microorganisms of interest, and the applicable regulations or standards. Testing laboratories or industry organizations provide guidelines for testing procedures and acceptance criteria.
Testing
Read More
Inspection
Read More
Certification
Read MoreTesting Services
- Drugs & Pharmaceutical Testing
- Cosmetics & Essential Oils Testing
- Medical Devices Testing
- Ayush - Ayurvedic Drug Testing
- Food Products Testing
- Agri Commodities Testing
- Fertilizers and Soil Testing
- Animal Food & Feed Testing
- Water - Drinking Water & Effluent Water Testing
- Industrial Oils and Lubricants & Petroleum Products Testing
- Coal & Coke and Solid Fuels Testing
- Ores & Minerals Testing
- Metals & Alloys Testing
- Plastics, Polymer, Rubber & Rubber Products Testing
- Resins & Adhesives Testing
- Paints, Varnish, Pigments & Surface Coating Testing
- Glass & Ink, Paper & Pulp Testing
- Industrial & Fine Chemicals Testing
- Dyes, Acids and Solvents Testing
- Soaps, Detergents & Toiletries Testing
- Packaging & Packaging Products Testing
- Gold & Silver Assaying & Hallmarking
- Cement, Concrete & Building Materials Testing
- Pollution and Environmental Studies
- Microbiological Assays. Etc.,









